FAQ: common questions about FSMA 204 and compliance
The Food Safety Modernization Act (FSMA) 204 is focused on improving traceability in the food supply chain. Its introduction has raised several common questions from those in the food industry, here are the most frequently asked:
How will FSMA 204 improve food safety?
The regulation aims to make it easier and faster to trace contaminated products back to their source, minimizing the impact of foodborne illness outbreaks and improving overall food safety.
What are the key components of FSMA 204?
FSMA 204 is a new regulation by the US Food and Drug Administration (FDA). It mandates additional traceability record keeping requirements for certain foods to enable efficient and accurate tracing of food from source to tables. Affected companies need to assign or utilize the assigned Traceability Lot Code (TLC) linking it to Key Data Elements (KDEs) at Critical Tracking Events (CTEs).
Which foods are subject to FSMA 204?
The Food Traceability List (FTL) includes a wide range of foods including fresh and frozen seafood, cheeses, nut butter, and fresh fruit and vegetables. Check the complete Food Traceability List on the FDA website to see if your products are affected.
Who must comply with FSMA 204 regulations?
Any partner on the supply chain whose product ends up on a US consumer plate will be affected by FSMA 204. It applies to food growers, processors, distributors, wholesale and retailers who are involved with foods on the FDA’s Food Traceability List. Non-US companies that are part of the food supply chain may be affected if they handle, process, package or ship these items.
How does FSMA 204 affect small and mid-sized businesses?
Small or mid-scale businesses may be eligible for full or partial exceptions from FSMA 204 Food Traceability Rule. While an exemption may apply to your business, it is worth noting that most businesses handling food on the FTL must comply, regardless of size. To help you determine if your business may be eligible for an exemption the FDA has developed an FDA exemption tool.
What record keeping systems do companies need for compliance?
Companies must have systems that capture and store Key Data Elements (KDEs) related to Critical Tracking Events (CTEs) like growing, receiving, processing and shipping food. Records must be kept in digital format – such as an electronic sortable spreadsheet - to facilitate sharing within 24 hours of an FDA request, during an outbreak, recall or contamination event. Companies will need to adapt or upgrade their systems and traceability plans to meet these requirements.
What are the consequences of non-compliance with FSMA 204?
Failure to comply can result in regulatory actions. Non-compliance can also damage company reputation and consumer trust, particularly in cases where a food safety issue arises.
What is the timeline for compliance with FSMA 204?
Businesses have until January 20, 2026, to comply with FSMA 204 requirements.
What technology solutions can help my business meet FSMA 204 compliance?
There are a wide variety of technologies on the market to help businesses get ready for compliance.
- Multiple technologies exist such as barcodes, RFID, QR and Databar which can all be employed to meet new regulations and begin the digital journey of your business.
- GS1 Standards align seamlessly with FSMA 204 requirements, facilitating the recording of KDEs through standardized barcodes such as GS1-128 and DataBar, the two most relevant for the food industry.
- Blockchain and IoT systems provide accurate real-time data collect and secure reporting, accessible by all stakeholders along the supply chain.
To find the system that meets the needs of your business today and in the future, the right digital partner is crucial. A digital partner will work with you to explore any potential challenges of implementing digitalization and outline a plan for integration, training and ongoing support.
What are the benefits of digitalizing your system beyond FSMA 204?
- Faster recalls: digital records allow for swift and precise recalls
- Efficiency: automating data collection reduces manual errors and improves operational efficiency
- Real-time tracking: real-time tracking of products across the supply chain, improving quality control and inventory management
- Facilitate fast and simplified reporting: digital systems can instantly collect and collate data for audits, compliance reports and to enable real-time decision making
- Cost savings: minimize waste and optimize inventory, significantly reduce costs over time
How does FSMA 204 impact importers and exporters?
Exporters of foods covered under the FTL must maintain records in compliance with FDA standards if their products enter the US market. Businesses involved in importing or exporting foods on the FTL are responsible for ensuring that foreign suppliers comply with US traceability rules.
What documentation must be provided in the event of an FDA request?
Within 24 hours of an FDA request, companies must provide records of KDEs related to CTEs, along with any information required to understand these records. Records must be available in an electronic sortable format that allows for efficient tracing.
How does FSMA 204 integrate with current food safety practices like Hazard Analysis and Critical Control Points (HACCP)?
FSMA 204 complements HACCP and other food safety systems by adding a layer of traceability that ensures quick pinpointing of where contamination may have occurred in the supply chain. Companies may need to update their HACCP plans to reflect FSMA 204 traceability requirements.
How does FSMA 204 impact third-party logistics providers?
Logistics providers handling food products on the FTL are often included in CTEs such as shipping and receiving. Due to this involvement, they are required to maintain traceability records for the products they transport, store or distribute.
What are Key Data Elements (KDEs) and Critical Tracking Events (CTEs)?
- KDEs: are specific data points that must be recorded at each stage of production. For example: product identifiers such as batch codes or lot numbers; dates, production, shipping, expiry; locations, farm, processing facility, distribution center; quantities
- CTEs: are key stages or activities in a product’s journey through the supply chain where key data must be collected. For example: harvesting and growing, receiving, processing, shipping and point of sale.
What are traceability lot codes (TLC)?
Traceability lot codes are used to identify a ‘product lot’. A TLC is assigned when the food is initially packaged (for raw agricultural commodities not obtained from a fishing vessel), received by the first land-based receiver (for foods obtained from a fishing vessel), or processed. Once a TLC is assigned it must remain the same as the food moves through the supply chain, until it is processed into a new form where it can be assigned a new TLC. For a more detailed understanding of TLCs and how they are used to meet the FSMA 204 rule, follow this link.
What role do GS1 Standards play in FSMA 204 compliance?
GS1 Standards align with FSMA 204 requirements, facilitating the recording of KDEs through standardized barcodes such as GS1-128, DataBar.
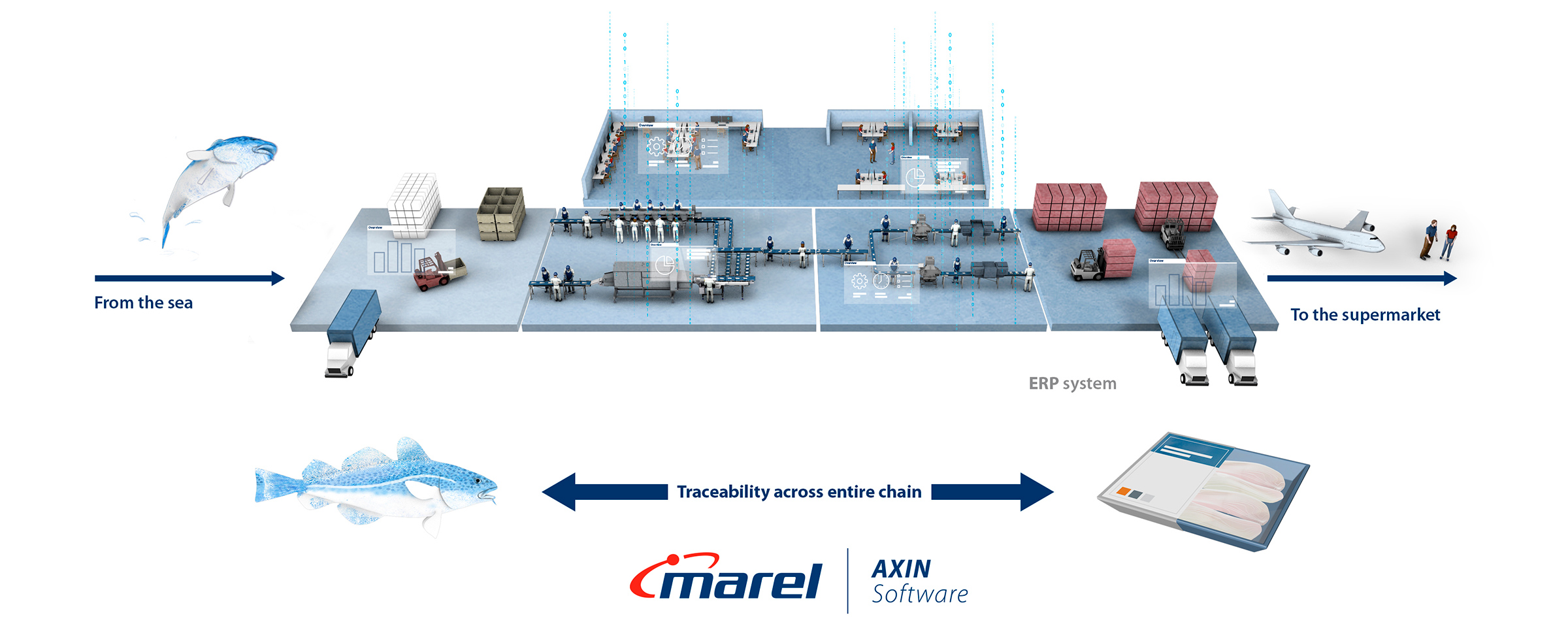
How can Marel help your business get ready for FSMA 204 ?
Marel offers a range of digital solutions that automate the collection of critical data, improve traceability, and streamline processes across the food supply chain. Marel’s traceability systems are a core part of our solutions and can be implemented as single data collection points, or an entire factory depending on your business needs. To ensure you are ready for any industry regulations, our traceability system follows GS1 Standards. With a team of experts who understand your industry and business, having Marel as a digital partner will ensure you can easily adapt to FSMA 204 requirements, ensuring compliance and improving overall efficiency.
Are you ready to explore the digital possibilities? Get in touch to learn how Marel solutions can help you prepare for FSMA 204 compliance or book a demo to see our systems in action.